hangover cures

Results
Study 1
Only data from the placebo condition (i.e., alcohol-only, without treatment) were considered. Analysis of the raw data revealed that n = 10 subjects did not report any hangover symptoms. Data from these n = 10 subjects were omitted. The results from the remaining n = 16 subjects are summarized in Table 1 and Figure1.
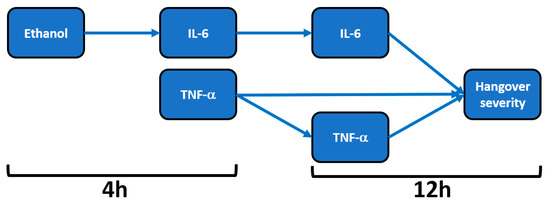
Figure 1. Relationship of hangover severity with blood ethanol concentration and the inflammatory response to alcohol. Note: Arrows represent significant correlations after bootstrapping. Abbreviations: Il = interleukin, TNF = tumor necrosis factor, h = hours after alcohol consumption. Data from Kim et al.

Table 1. Biomarkers of alcohol metabolism, the inflammatory response to alcohol consumption and their relation to hangover severity.
Mean (SD) hangover severity, assessed 12 h after alcohol consumption, was 16.2 (1.8). The analysis revealed no significant correlations between blood ethanol and overall hangover severity at any timepoint. Also, no significant correlations were found between blood acetaldehyde and overall hangover severity at any timepoint. Significant correlations were found between hangover severity and TNF-α at 4 h (rB = 0.679, BCa 95%CIB = 0.440, 0.835) and TNF-α at 12 h (rB = 0.625, BCa 95%CIB = 0.252, 0.889). A significant correlation was also found between IL-6 at 12 h and hangover severity (rB = 0.459, BCa 95%CI = 0.036, 0.796).
On the contrary, the inflammatory response seems to be directly related to blood ethanol concentrations. That is, significant positive correlations were found between blood ethanol at 4 h and IL-6 at 4 h (rB = 0.482, BCa 95%CIB = 0.038, 0.803) and between blood ethanol at 4 h and IL-6 at 12 h (rB = 0.573, BCa 95%CIB = 0.216, 0.835). Correlations between IL-6 and TNF-α did not reach statistical significance for any timepoint combination. In contrast to ethanol, the inflammatory response was not significantly associated with acetaldehyde concentrations.
3.2. Study 2
Data on hangover severity of three subjects were not collected. Raw data from the remaining n = 13 subjects were used for the statistical analysis. Their mean (SD) hangover severity, assessed 14 h after alcohol consumption, was 4.4 (0.5). The results of the assessments in the alcohol-only condition are summarized in Table 2.

Table 2. Biomarkers of alcohol metabolism, oxidative stress and the inflammatory response to alcohol consumption and their relation to hangover severity.
Acetaldehyde levels significantly increased directly after alcohol consumption (p < 0.0001), but declined steadily thereafter, and at 12 h after drinking, acetaldehyde could no longer be detected, whereas ethanol concentrations were still detectible after this time period. No significant correlations were found between hangover severity and blood ethanol or acetaldehyde concentrations at any time point after alcohol consumption.
CRP concentration at 4 h was significantly associated with hangover severity at 14 h (rB = 0.534, BCa 95%CIB = 0.120, 0.825). No significant correlations between IL-6 and hangover severity were found at any time point, and the associations between blood ethanol and acetaldehyde concentrations and IL-6 were also not significant at any timepoint. A significant correlation was found between IL-6 at 2 h and CRP at 4 h (rB = 0.587, BCa 95%CIB = −0.874, −0.130). CRP concentrations did not correlate significantly with blood ethanol or acetaldehyde concentrations at any timepoint.
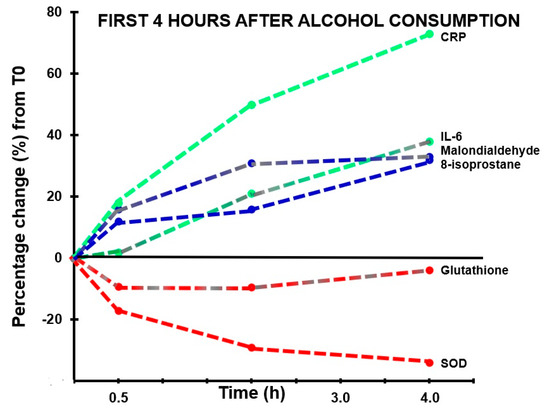
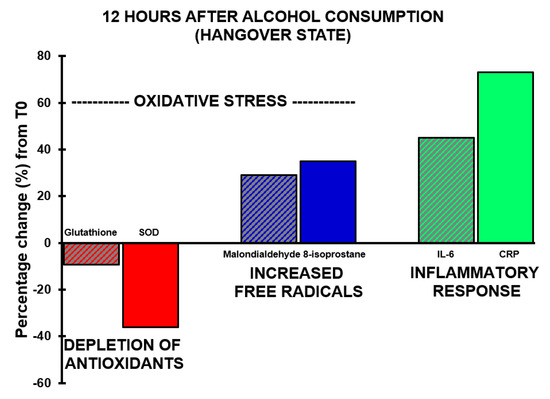
With regard to antioxidants, the assessments revealed that both glutathione and SOD concentrations showed a decrease after alcohol consumption, but at no timepoint did their concentrations correlate significantly with hangover severity. The concentration of 8-isoprostane increased over time and reached its highest point 12 h after alcohol consumption (p < 0.0001).
A significant correlation was found between hangover severity and 8-isoprostane at 12 h (rB = 0.475, BCa 95%CIB = 0.06, 0.78). Also, the blood malondialdehyde concentration at 0.5 h correlated significantly with hangover severity at 14 h (rB = −0.707, BCa 95%CIB = −0.96, −0.16). The negative correlation suggests that higher malondialdehyde concentrations directly after drinking are associated with experiencing less severe hangovers. 8-isoprostane at 2 h after alcohol consumption correlated significantly, and negatively, with acetaldehyde assessed 0.5 h (rB = −0.612, BCa 95%CIB = −0.884, −0.192) and 2 h (rB = −0.613, BCa 95%CIB = −0.881, −0.191) after alcohol consumption, while ethanol concentrations revealed non-significant positive associations at these timepoints. Assessments at 2 h after alcohol consumption revealed a significant negative association (rB = −0.541, BCa 95%CIB = 0.113, 0.833) between oxidative stress (8-isoprostane) and antioxidant depletion (glutathione). The results are summarized in Figure 2 and Figure 3.
Figure 2. Markers of oxidative stress and inflammatory responses in the first four hours after alcohol consumption. Percentage changes between assessments made in the first 4 h after alcohol consumption relative to T0 (the assessment made before alcohol consumption) are shown. Abbreviations: CRP = C-reactive protein, IL-6 = interleukin-6, SOD = superoxide dismutase.
Figure 3. Oxidative stress and the inflammatory response during alcohol hangover. Percentage changes between assessments made 12 h after alcohol consumption relative to T0 (the assessment made before alcohol consumption) are shown. Abbreviations: CRP = C-reactive protein, IL-6 = interleukin-6, SOD = superoxide dismutase.
4. Discussion
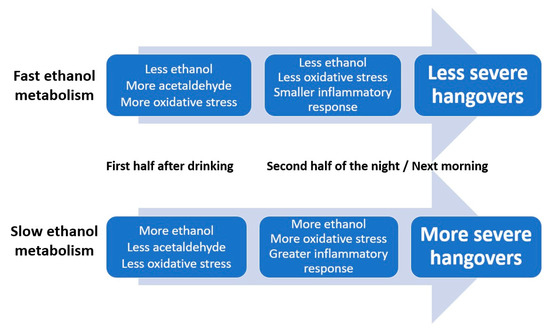
Multiple lines of evidence, as summarized elsewhere , suggest that the amount of ethanol present in the blood is an important determinant of hangover severity. Specifically, faster conversion of ethanol into acetaldehyde and other aldehydes is associated with having less severe hangovers . Data from the two studies evaluated here confirm and extend previous findings. For example, the data support previously reported relationships between oxidative stress and inflammatory responses to alcohol consumption (i.e., increases in IL-6 and CRP). Blood malondialdehyde concentration at 0.5 h correlated negatively with hangover severity at 14 h. Thus, higher oxidative stress in the first hours after drinking was associated with less severe next-day hangovers. The significant positive correlation between hangover severity and 8-isoprostane at 12 h after alcohol consumption suggests that more oxidative stress, experienced at a later stage after alcohol consumption, is associated with having more severe hangovers. In other words, a quick ethanol metabolism results in more oxidative stress in the first hours of drinking. This is associated with having less severe next-day hangovers. On the other hand, if ethanol metabolism is relatively slow, there will be more oxidative stress the following morning, which is associated with having more severe hangovers. Increased ethanol concentration, but not acetaldehyde, was associated with elevated IL-6 levels. The magnitude of the inflammatory response correlated significantly with hangover severity.
Low-grade inflammation, i.e., relative minor elevations in proinflammatory cytokine concentrations, is a common underlying condition in many diseases . Alcohol consumption is one of the many factors that can provoke such an elevation of proinflammatory cytokines. The studies reviewed in the introduction, and the data from the two studies presented here, demonstrate significant and robust elevations in cytokine concentrations, including IL-6, IL-10, IL-12, IFN-γ and TNF-α, after alcohol consumption and during the hangover state. The relationship between CRP and cytokine production is complex and bi-directional, and CRP production is usually followed in time by cytokine production, particularly IL-6 and TNF-α are induced by the presence of CRP .
Results of the two studies were in agreement that an inflammatory response follows alcohol consumption. However, there were also differences. For example, whereas Kim et al. found a significant correlation between Il-6 and hangover severity, this correlation did not reach significance in the study by Mammen et al. Other studies also reported no significant correlation between IL-6 concentration and hangover severity . The finding by Mammen et al. that CRP concentration did correlate significantly with hangover severity is in line with previous findings . In contrast to other studies [, we did not find significant correlations between hangover severity and blood ethanol concentration. The observed differences between the outcomes of the two studies and available literature can be explained by differences in the study designs. For example, with regards to subject recruitment, one study took genetic polymorphism into account whereas the other study did not . The administered type and amount of alcohol differed between the two studies, which resulted in different blood alcohol concentrations. At 4h after alcohol consumption, the difference in blood alcohol levels was roughly 32.1 mg/dL (~0.03%) between the two studies, which might explain why some associations with overall hangover severity were only significant in one study. Further, different hangover assessment scales were used, and the assessments of hangover severity were made at different points in time after drinking (12 versus 14 h after drinking). Similarly, the biomarkers of alcohol metabolism and oxidative stress were assessed at various points in time after alcohol intake, and the timing of the assessments differed between the two studies. Of note, the expression of various cytokines is interrelated and time-dependent. Therefore, different timing of assessments may explain why in some study designs, a relationship of a biomarker of alcohol metabolism or specific cytokines significantly correlated with hangover severity, while in another study, the same correlation does not reach statistical significance. Notwithstanding this, it is clear from all studies that alcohol consumption is followed by oxidative stress and an inflammatory response.
There are several issues that deserve attention when interpreting the results of the two studies we re-analyzed here. First, participants of the studies were young males only. Therefore, it is unclear to what extent the results can be generalized to females and other age groups. Research has shown that age and sex differentially impact both alcohol elimination rate and inflammatory responsiveness . Other studies that did include both male and female subjects (discussed in the introduction section of this article) did not report any sex or age differences in aspects of the pathology of the alcohol hangover. However, these aspects may not have been reported as the investigated samples were not powered to conduct such comparisons. Future research should therefore be conducted in samples comprising both men and women, and also investigate other age groups. These studies should have sufficient sample size to allow comparisons among groups.
Second, the subjects in the re-evaluated studies were of Asian descent. There are differences reported in ethanol metabolism between different ethnic groups. For example, it has been found that in populations of Asian descent, subjects with ALDH2*2 alleles, i.e., those who breakdown acetaldehyde more slowly, typically report significantly worse hangovers, and are more likely to experience hangovers at relatively lower alcohol consumption levels. Therefore, future studies should confirm the current observations in subjects of non-Asian descent.
Third, the administered amount of alcohol consumed was relatively low in the re-evaluated studies. It would be interesting to further investigate the observed effects when higher amounts of alcohol are consumed that may better reflect real-life alcohol consumption. On the other hand, also with the currently applied consumption levels, inflammatory effects were evident. Moreover, recent studies have demonstrated that consuming large amounts of alcohol is not a necessity for having hangovers . In fact, a recent large sample study revealed an average estimated BAC of 0.03% of subjects that reported a hangover , which is lower that the achieved BACs in the re-evaluated studies here.
Fourth, both studies assessed hangover severity using a composite scale of hangover symptoms. Recent examinations revealed that the magnitude of hangover severity of composite scales ultimately depends on which symptoms are included in such scales . This may have had an impact on the correlational analyses. Future research should also use single-item hangover severity assessments as these better reflect overall hangover severity, and its impact. It is also important to thoroughly investigate whether potential participants of hangover studies will likely have a hangover when a certain amount of alcohol is consumed. In the study by Kim et al, n = 10 subjects were excluded after participation because they reported no hangover during the study. Instead of excluding them after participation, it is more ideal to identify them during screening, and screening tools for this purpose are currently under development.
To conclude, it should be noted that the current body of data examining the pathology of the alcohol hangover is limited, and much more research is needed to further elucidate the exact nature of how ethanol metabolism, oxidative stress and the immune response are interrelated and may impact hangover severity. In this context, it is important to note that the two studies in this article comprised only male participants. As it is known that ethanol elimination rate is influenced by sex, future studies should be conducted to further investigate the role of the immune system on hangover in samples comprising both men and women, of different ethnicity and age.
Taken together, the timing of the observed assessments (see Figure 2 and Figure 3) suggests that initial slow elimination of ethanol in the first hours after drinking results in more ethanol present in the second half of the night and the next morning, which will elicit more oxidative stress and a stronger inflammatory response. Together, these processes result in having more severe next-morning hangovers (See Figure 4).